 GenSpera, Inc., a leader in developing prodrug therapeutics for the treatment of cancer, has announced that the United States Patent and Trademark Office (USPTO) issued the patent “Tumor Activated Prodrugs” for GenSpera’s G-202, the company’s lead drug candidate.
GenSpera, Inc., a leader in developing prodrug therapeutics for the treatment of cancer, has announced that the United States Patent and Trademark Office (USPTO) issued the patent “Tumor Activated Prodrugs” for GenSpera’s G-202, the company’s lead drug candidate.
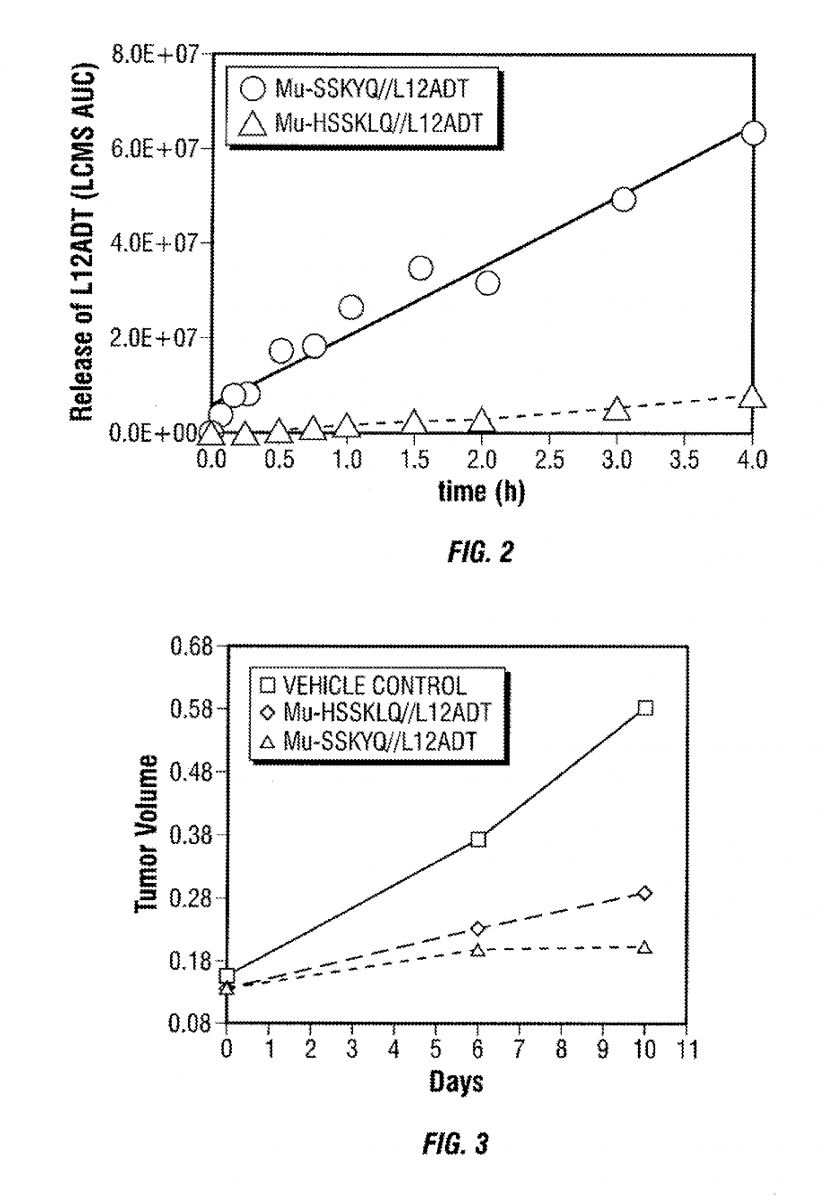
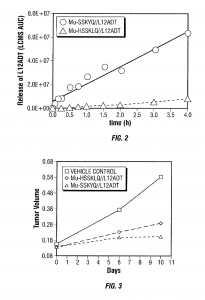
This patents covers the development of G-202, a prodrug activated by Prostate Specific Antigen (PSA) and designed to deliver a thapsigargin (toxin) derivative selectively to prostate cancers.
Researchers have isolated thapsigargin from the plant Thapsia garganica. This component inhibits the sarco/endoplasmic reticulum Ca2+ ATPase and induces cells to undergo apoptosis.
By conjugating thapsigargin to peptides which are only substrates for either prostate specific antigen (PSA) or prostate specific membrane antigen (PSMA), researchers could create prodrugs, a type of drug that alone does not have any pharmacological effect, but in the body is transformed into the active therapeutic. Once conjugated with the specific peptide, the drug can be targeted towards the malignant tissue, and upon internalization it releases the selected toxin, killing tumor cells.
G-202 or mipsagargin, is a derivative of thapsigargin (12ADT) and is currently in Phase II clinical trials for glioblastoma and hepatocellular carcinoma for patients who have undergone sorafenib (Nexavar®) therapy.
GenSpera already owns or licenses 13 issued patents overall, 10 of them involve prostate cancer.
“The issuance of this patent further strengthens our intellectual property position for targeted anti-cancer prodrugs and recognizes their unique applicability in treating prostate cancer. Each year over 230,000 men are diagnosed with prostate cancer in the United States alone and approximately 30,000 men die of the disease. The market for prostate cancer drugs is estimated to be $6.7 billion by the year 2020. Current treatment options are dominated by drugs that work via chemical castration of the patient. It is important that we develop safe and effective drugs for these men without the very significant side effects of current treatments,” Craig Dionne, PhD and GenSpera CEO stated in a PR Newswire press release.