A Phase 1 clinical trial of Harpoon Therapeutics’ immunotherapy candidate, HPN424 for metastatic castration-resistant prostate cancer (mCRPC), has treated the first patient, according to the company.
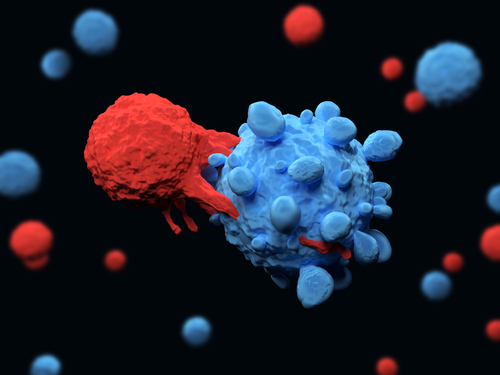
HPN424 uses the Harpoon’s TriTAC (Tri-specific T cell Activating Construct) platform. This approach is intended to work as an adaptor, connecting the patient’s own immune T-cells with tumor cells.
This is possible because TriTAC products target cancer cells and immune cells at the same time, bringing them together. HPN424, particularly, is a bi-specific antibody (aka T-cell engager) that binds the PSMA protein — found in more than 90 percent of mCRPC lesions — and the CD3 molecule on T-cells.
Harpoon designed HPN-424 to be the smallest T-cell engager to penetrate solid tumors with superior effectiveness, last longer in the blood, and recruit patients’ T-cells to kill malignant cancer cells.
The multicenter, international, open-label study (NCT03577028) will assess the safety, tolerability and pharmacokinetics (the study of a compound’s absorption, distribution, metabolism, and excretion) of HPN424 in approximately 40 adult patients with mCRPC.
Patient recruitment is underway at the Sarah Cannon Research Institute, in Nashville, Tennessee. (More information and contacts here.) The study is expected to end by December 2020.
“We are pleased to initiate Harpoon’s first clinical trial with HPN424 in patients with advanced prostate cancer, an important milestone that marks our transition to a clinical-stage company,” Jerry McMahon, PhD, Harpoon’s President and CEO, said in a press release.
“HPN424 is the first TriTAC compound to enter clinical testing and represents a novel class of T cell therapeutics,” he added. McMahon also said that results are expected in 2019, and should provide the information needed to determine the optimal HPN424 dose and treatment regimen for the company’s other planned trials in prostate cancer.
In this trial’s first phase, the investigational therapy will be administered once weekly via intravenous infusion at increasing doses until a therapeutic dose level is reached. In the second phase, Harpoon will use the selected dose to further analyze the safety and effectiveness of HPN424. Additional groups of 18 patients each may be added.
“Immunology represents a new frontier for the treatment of prostate cancer,” said Charles G. Drake, MD, PhD, director of genitourinary oncology at New York Presbyterian/Columbia University Medical Center and a Scientific Advisory Board member at Harpoon.
“HPN424, an innovative biologic designed to activate T cells in the tumor, is highly potent in killing prostate cancer cells in preclinical models and could be a promising option for these men with advanced disease,” added Drake, also the associate director for clinical research at the Herbert Irving Comprehensive Cancer Center.
“Pharmacological insights gleaned from the study should help inform development of both HPN424 and our pipeline of additional products,” Law said.
Besides HPN424, Harpoon is developing other TriTAC-based compounds for multiple myeloma, small cell lung cancer, and other cancers.