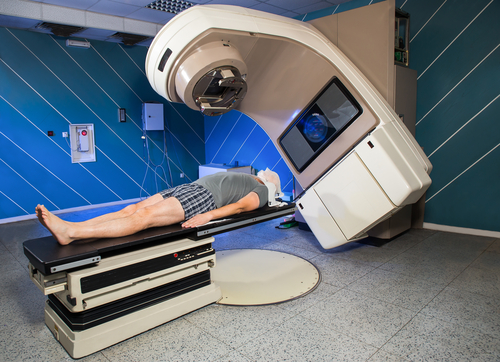
Physicians at University Hospitals Seidman Cancer Center are conducting a clinical trial that uses a novel form of stereotactic body radiotherapy (SBRT) to deliver radiation to the exact area affected by prostate cancer, instead of irradiating the entire gland. This trial aims to determine if using radiation only on a targeted region in an early stage prostate cancer will increase treatment options and reduce side effects.
If successful, the trial (NCT02163317), which uses a patented, magnetic resonance (MRI)-guided approach to SBRT, could impact the standard of care for patients with low- or intermediate-risk prostate cancer.
Stereotactic body radiotherapy (SBRT), also called stereotactic ablative radiotherapy (SABR), is a type of radiation therapy in which small, well-defined tumors are treated with high doses of radiation by focused millimeter precision. The goal of this type of focused radiation therapy is to administer a high enough dose to kill the cancer cells while lessening damage to the surrounding organs and tissues.
The first patient had the procedure preformed by Dr. Lee Ponsky, a urologist at the UH Urology Institute, and by Dr. Rodney Ellis, a radiation oncologist and the trial’s co-investigator. To date, no side effects have been reported.
“Our hypothesis is that this method may offer a viable treatment option for men with early stage prostate cancer before their cancer advances, while also minimizing or even eliminating life-changing side effects of treatment,” Dr. Ellis, vice chairman, Clinical Affairs, Radiation Oncology, at the UH center in Cleveland, Ohio, said in a news release. “We want to know if response and cure rates using focal radiotherapy within the prostate can achieve two goals: the first, to successfully eliminate localized malignancies when the disease is in extremely early stages, and second, to reduce the treatment’s impact on a patient’s quality of life.”
According to The National Cancer Institute, 80 percent of all prostate cancers are identified in early stages, when the lesions are confined to the prostate.
Patients diagnosed at this stage can opt to go on “active surveillance,” where the prostate is regularly monitored, or for whole gland therapy either with surgery or radiation therapy. However, whole prostate radiotherapy has a substantial risk of side effects, including erectile dysfunction and rectal bleeding.
For patients with lesions amenable to focused radiotherapy, this trial offers a minimally invasive treatment approach that can be completed in three sessions over a period of a week.
“Prostate cancer remains the sole malignancy where treatment is always delivered to the whole organ in routine practice,” said Mitchell Machtay, MD, chairman of the Department of Radiation Oncology at UH Case Medical Center and Case Western Reserve University School of Medicine, and the study’s principal investigator. “It is time to investigate new and better options for low-risk prostate cancer patients. Radiology advancements in ultrasound, CT, and MRI enable us to accurately identify, measure, and target malignancies more precisely than ever before.”
The clinical trial will enroll 12 patients selected using a University Hospitals’ patented methodology, developed in part by Dr. Ellis. The new system is licensed by Philips Medical Systems, which is assisting in the trial along with Invivo and Elekta.