SpaceOAR hydrogel, which protects organs against damage from radiation therapy, has been administered for the first time to a Canadian prostate cancer patient, according to its maker, Augmenix.
Doctors prescribed the treatment at the University Health Network in Toronto’s Princess Margaret Cancer Centre. A hydrogel is a liquid that changes to a solid.
“Recent clinical data show that SpaceOAR hydrogel helps to significantly reduce the risk of rectal and urinary toxicities and loss of sexual function associated with radiation therapy in the treatment of prostate cancer,” John Pedersen, CEO of Augmenix, said in a press release. “We are pleased that the first patient to be treated with SpaceOAR hydrogel in Canada took place at the Princess Margaret Cancer Centre, which is globally recognized for innovation and excellence in cancer care.”
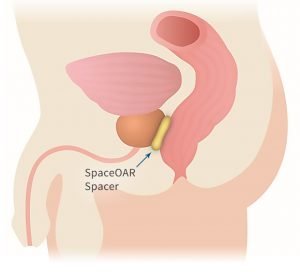
While many advancements have been made in prostate cancer radiotherapy, the proximity of the prostate and rectum makes it difficult to deliver radiation to the prostate without harming the rectum. That makes the rectum what doctors call an Organ At Risk (OAR) in this case.
Doctors insert the hydrogel on the outer wall of the rectum to protect it from radiation. It changes from a liquid to a solid, pushing the prostate and rectum apart. It stays in the body for three months during radiation therapy, and is gradually absorbed and eliminates after that.
Augmenix did a Phase 3 clinical trial (NCT01538628) to assess whether SpaceOAR could reduce radiation damage to the rectum. The study also assessed its safety.
Prostate cancer patients treated with SpaceOAR had lower radiation levels in their rectum, better urinary function and better sexual function three years after treatment than those who did not receive it, according to results that Augmenix released in January.
Those who received the therapy had 73.5 percent less radiation in their rectum and 49 percent less in the tip of their penis than those who didn’t.
Researchers also evaluated patients’ wellbeing by measuring bowel, urinary, and sexual quality of life. Three-years after radiotherapy, patients who received SpaceOAR were eight times less likely to experience significant declines in their quality of life, compared with untreated men.
Two-thirds of men who were sexually active before receiving SpaceOAR treatment could have erections three years later, compared with 37.5 percent of untreated men.
Princess Margaret researchers are working on a pilot study that will evaluate SpaceOAR’s ability to treat prostate cancer in 20 patients.
“The risk of side effects, particularly those affecting the bowel, are key considerations for both clinicians and patients in the decision-making in the treatment of prostate cancer,” said Peter Chung, radiation oncologist at Princess Margaret and assistant professor in the Department of Radiation Oncology at the University of Toronto. “Our team at Princess Margaret looks forward to using SpaceOAR hydrogel as indicated as part of our ongoing efforts to bring patients the most effective treatment options while reducing the side effects of radiation therapy.”
The U.S. Food and Drug Administration approved SpaceOAR in 2015. It was the first spacer therapy it had approved to protect the rectum of prostate cancer patients undergoing radiotherapy.

